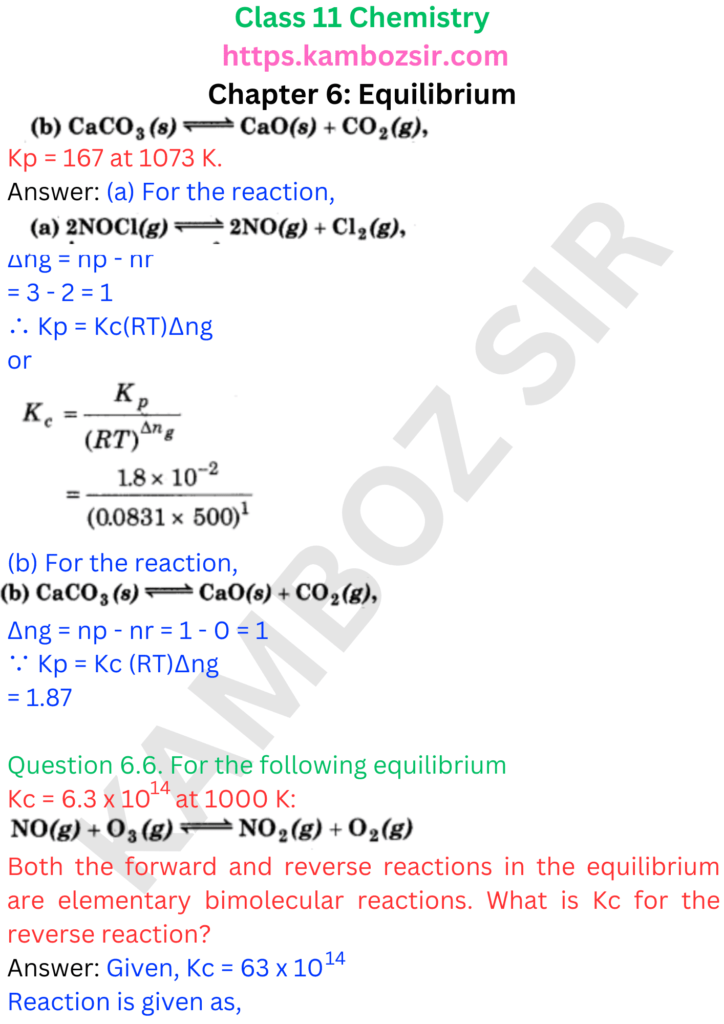
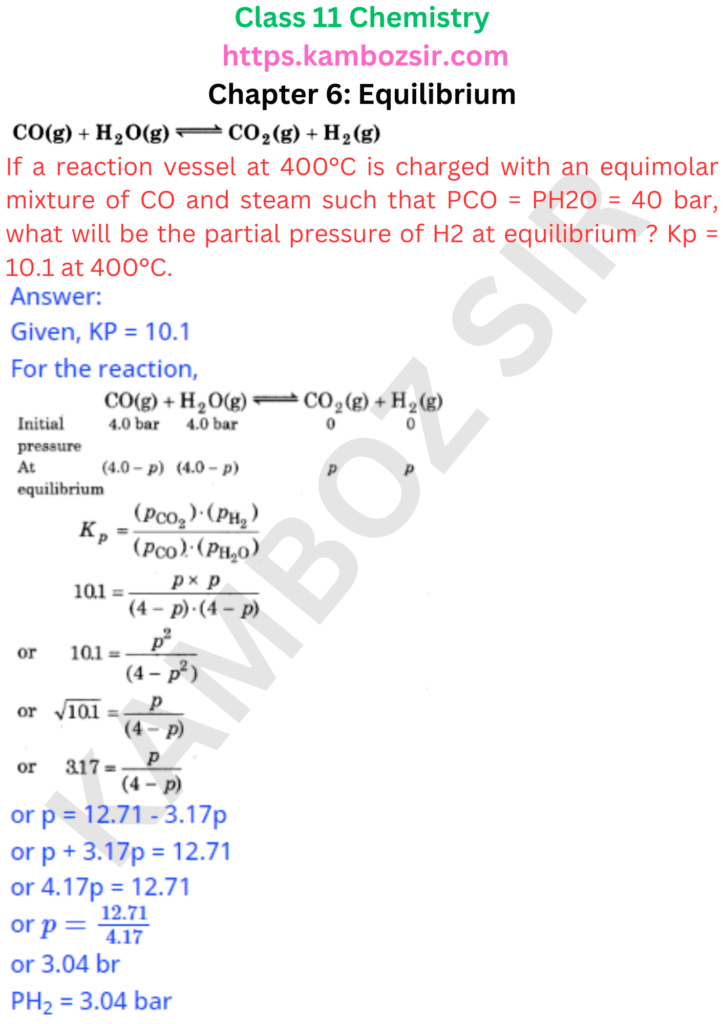
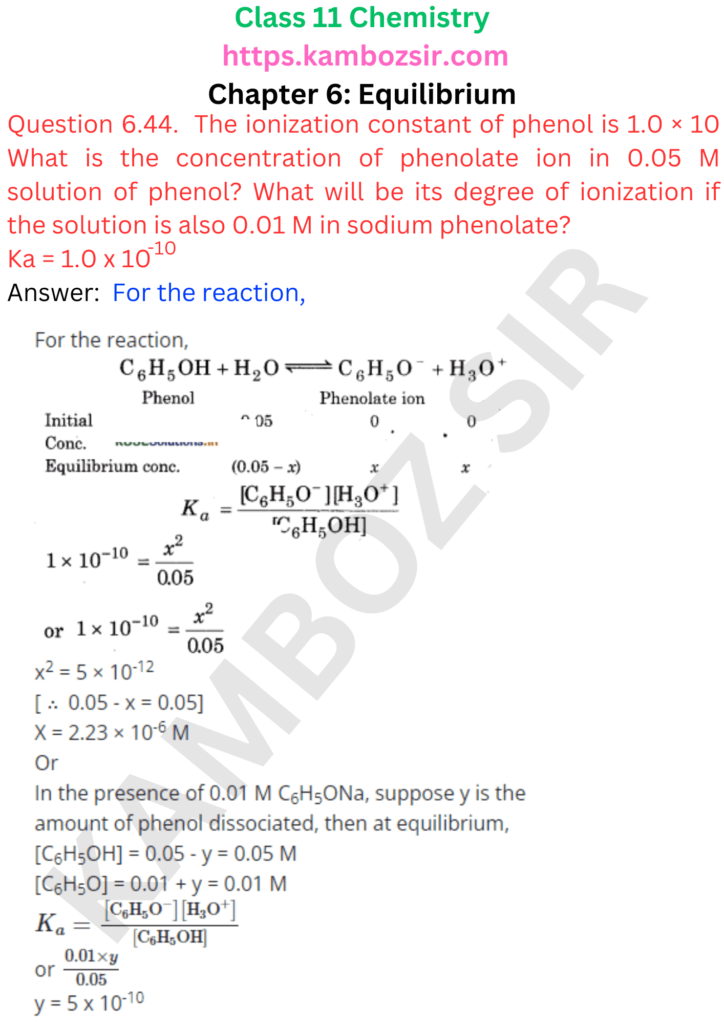
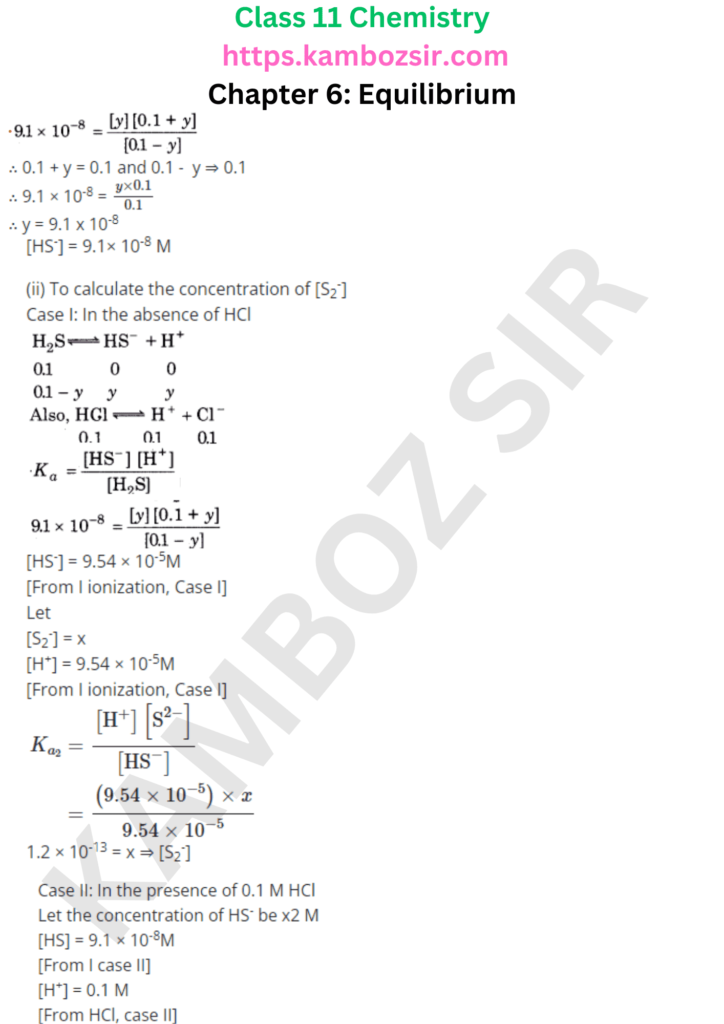
Class 11th Chemistry Chapter 6: Equilibrium Solution
Class 11th Chemistry Chapter 6: Equilibrium Solution
In this chapter, we will explore the fascinating concept of equilibrium in chemical systems. Equilibrium is a state of balance where the forward and reverse processes of a chemical reaction occur at equal rates. We will study the factors that influence the attainment of equilibrium and how it can be altered.
We will begin by understanding the concept of dynamic equilibrium, where the rates of the forward and reverse reactions are equal. We will learn about the characteristics of a system at equilibrium and the significance of the equilibrium constant (K) in determining the extent of a chemical reaction.
Next, we will delve into the principles of Le Chatelier’s principle, which states that when a system at equilibrium is subjected to a change in conditions, it will respond in a way that minimizes the effect of that change. We will explore the effects of changes in concentration, pressure, and temperature on the equilibrium position.